Multiple Choice
Identify the choice that best completes the statement or answers the question.
Identify the choice that best completes the statement or answers the question.
1.
Convert 475 nanometers to cm.
a. | .475 cm | c. | 4.75 x 10-5 cm |
b. | 475 cm | d. | 4.75 x 109 cm |
2.
What is the approximate diameter of a metal atom (Fe for example)
a. | 2.8 cm | c. | 2.8 Å (angstroms) |
b. | 2.8 nm | d. | 280 nm |
3.
Which of the following elements is a nonmetal?
a. | Li | c. | Ti |
b. | Sr | d. | N |
4.
3416S2- has ____ protons,
_______neutrons_________ electrons
a. | 34, 16, 2 | c. | 16, 18, 18 |
b. | 16, 34, 18 | d. | 16, 16, 16 |
5.
The name of the compound Fe2S3 is
a. | diferrum trisulfur | c. | triiron sulfate |
b. | iron(II) sulfur(III) | d. | iron (III) sulfide |
6.
The chemical formula for potassium sulfate is
a. | P2S | c. | KSO3 |
b. | K2S | d. | K2SO4 |
7.
An experiment calls for 20.0 g of n-propyl alcohol , a substance whose density
is 0.8 g/mL. What volume of n-propyl alcohol should be used?
a. | 20.0 mL | c. | 16 mL |
b. | 25.0 mL | d. | 16 g |
8.
What is the mass, in grams, of 0.100 mol Ba(NO3)2
a. | 0.100 g | c. | 261.3 g |
b. | 26.13 g | d. | 2.61 g |
9.
The number of oxygen atoms in 0.34g of H2O2 is ________
atoms.
a. | 1.2 x 1022 | c. | 1.2 x 1023 |
b. | 6.02 x1023 | d. | 3400 |
10.
When the following is balanced, what is the coefficient in front of the
oxygen?
___C5H10 +___ O2 -->____ CO2 +____ H2O
___C5H10 +___ O2 -->____ CO2 +____ H2O
a. | 5 | c. | 15 |
b. | 10 | d. | 29 |
11.
What is the weight percent nitrogen in ammonium nitrate
(NH4NO3)
a. | 25 % | c. | 82% |
b. | 17 % | d. | 35 % |
12.
A 100.0 g sample returned the following analyses: 40.2 % K, 26.9% Cr and 32.9%
O. What is the simplest empirical formula of this compound?
a. | KCrO | c. | K2CrO4 |
b. | Cr3OK | d. | K2Cr2O7 |
13.
A double displacement reaction between Pb(NO3)2 and
CaCl2 will produce what products:
a. | PbO2 and CaClO2 and N2 | c. | CaPb and 2Cl2NO3 |
b. | PbO and CaCl2 and NO5 | d. | PbCl2 and Ca(NO3)2 |
14.
In a single displacement reaction Ag + HCl --> AgCl + H+ in an aqueous environment How many grams of HCl would be required to just react with 10.7 grams of Ag.
a. | 10.7 grams HCl | c. | 3.62 g HCl |
b. | 12.0 M HCl | d. | 0.1 mole HCl |
15.
Given the following : 3NO2(g) + H2O(l) ---> 2HNO3(aq) + NO(g).
What is the number of moles of HNO3 that can form when 3.0 mol NO2 and 3.0 mol H2O react?
a. | 6 | c. | 3 |
b. | 2 | d. | 4 |
16.
A chemical reaction that releases heat energy to the environment is __________
with a ____________ enthalpy of reaction
a. | exothermic, negative | c. | exothermic, positive |
b. | endothermic, negative | d. | endothermic, negative |
17.
Which of the following would not conduct electricity
a. | molten NaCl | c. | aqueous CuSO4 |
b. | hexane | d. | 3.0 M HCl |
18.
Which of the following will depress the freezing point of water.
a. | heating the water first. | c. | raising the pressure 10 Torr. |
b. | adding an ionic substance. | d. | lowering the pressure 10 Torr. |
19.
Which is the lowest energy electron orbital?
a. | 1s | c. | 3d |
b. | 2s | d. | 1p |
20.
What is the correct electron configuration for gallium (Ga)?
a. | [Kr] 3p3 | c. | 1s2,2s2,3s2,4s2,4d10, 4p1 |
b. | [Ar]4s2,3d10, 4p1 | d. | [Ga] 4p1 |
21.
If a sample of H2 occupies 3.00 L at 640 Torr and 24 C, what volume
would it occupy at STP?
a. | 2.00 L | c. | 1.92 L |
b. | 3.5 L | d. | 2.32 L |
22.
How many unpaired electrons are present in O2-
a. | 0 | c. | 6 |
b. | 2 | d. | 8 |
23.
How many moles of H2S gas would be present in a cylinder with a
volume of 2.00 L at a pressure of 2.00 atmospheres at 200 K R= 0.0821 L atm / mol K
a. | 0.24 mol | c. | 0.273 mol |
b. | 2.00 mol | d. | 1.6 x 10 -3 mol |
24.
Which of the following atoms has the largest radius?
a. | Fe | c. | Fe 3+ |
b. | Fe 2+ | d. | None of the choices. All are same. |
25.
Which of the following has the greatest electronegativity?
a. | F | c. | P |
b. | O | d. | Br |
26.
Which of the following would have the largest radius ?
a. | Be | c. | C |
b. | N | d. | F |
27.
Electronegativity for elements on the periodic table (excluding the Noble
Gases) increases ________ and ___________.
a. | down and right | c. | up and right |
b. | down and left | d. | up and left |
28.
The chemical bonding between Mg and O in MgO is best described as
_________ (based on differences in electronegativity).
a. | complex coordinate | c. | covalent |
b. | ionic | d. | polar covalent |
29.
According to VSEPR theory the expected molecular geometry of PH3 is
a. | linear | c. | trigonal pyramidal |
b. | trigonal planar | d. | tetrahedral |
30.
The Lewis dot structure for carbon monoxide is best characterized by
a. | not obeying octet rule | c. | a triple bond |
b. | 2 double bonds | d. | single bonds only |
31.
How many sigma bonds and pi bonds are present in the following structure
?
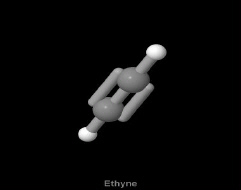

a. | 3 sigma, 2 pi | c. | 4 sigma |
b. | 3 pi, 2 sigma | d. | 2 sigma, 2 pi |
32.
Which of the following has a bent structure with approximately a 109 degree bond
angle
a. | SO4 -2 | c. | CO |
b. | H20 | d. | CO2 |
33.
How many grams of KNO3 must be added to make 250 mL of solution at
0.2 M
a. | 250 g | c. | 5.05 g |
b. | 101. g | d. | 0.2 g |
34.
How many mL of 0.1 M KOH would be need to exactly neutralize 10 mL of 0.1 M
H3PO4
a. | 10 mL | c. | 30 mL |
b. | 100 mL | d. | 1 mL |
35.
At some temperature the Keq for the decomposition of
N2O4(g) --> 2 NO2(g) is 2.50 x 10-3 If the
equilibrium concentration of N2O4 is 0.100 M what is the concentration of
NO2?
a. | 0.100 M | c. | 0.0 M |
b. | 0.016 M | d. | 2.5 x10-4 M |
36.
Which of the following is the weakest acid?
a. | ascorbic acid, HC6H7O6 (Ka = 8.0 x 10-5) | c. | acetic acid, HC2H3O2 (Ka = 1.8 x 10-5) |
b. | nitrous acid HNO2 (Ka = 7.1 x 10-4) | d. | hypobromous acid HBrO (Ka = 2.05 x 10-9) |
37.
If the pH of a 0.01M solution of acid is 2.0 the Ka must be
a. | a strong acid | c. | a weak acid |
b. | a strong base | d. | a weak base |
38.
A 1.0 M solution of KOH would have a pH of
a. | 1 | c. | 0 |
b. | 13 | d. | 14 |
39.
Which of the following represents a conjugate acid-base pair?
a. | HCl, NaOH | c. | H2SO4, SO4-2 |
b. | HNO3, NO3- | d. | NH4Cl, NH4+ |
40.
Which of follow corresponds to the formula for propane
a. | C3H3 | c. | C2H5OH |
b. | C3H8 | d. | C3H6 |
41.
Which of the following would have the highest boiling point ?
a. | HF | c. | HBr |
b. | HI | d. | HCl |